“All-in-One Certificate” ushers Shandong medical devices into the era of universal surgical care kits.
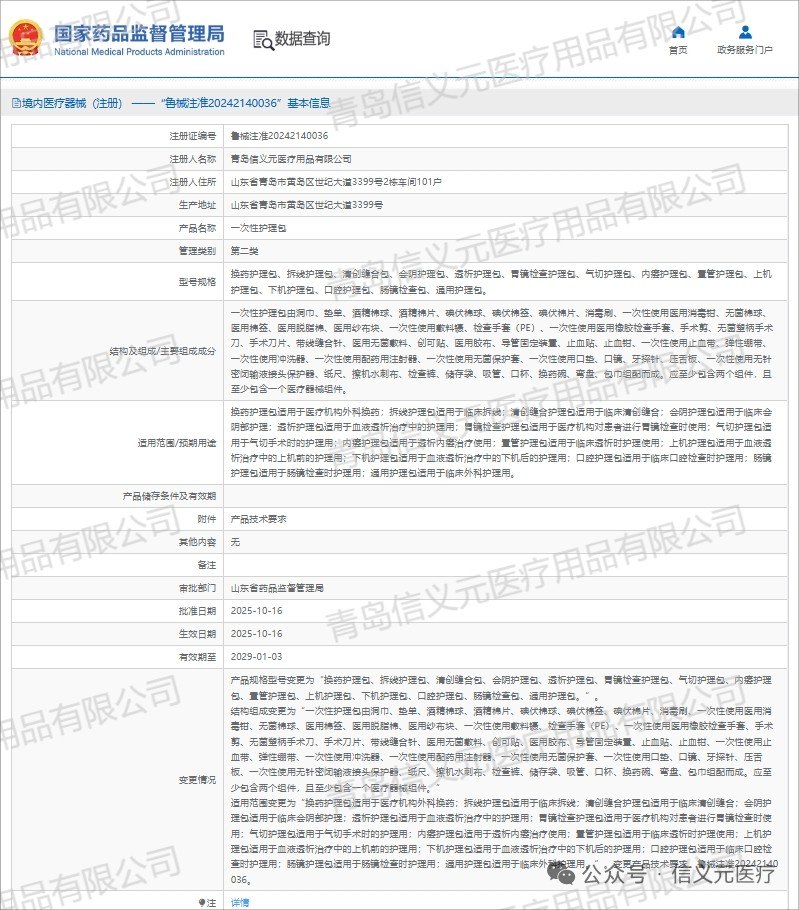
Breakthrough Progress: From “Multiple Certificates for Different Departments” to “One Certificate
for Universal Use”.
Traditional single-use nursing kits and surgical kits required separate medical device registration certificates
for different departments (such as ophthalmology, obstetrics, surgery, etc.) and applications, resulting in
numerous certificates and complex management. Our company's innovative breakthrough successfully
integrates multi-department applicable products under a unified technical framework through optimized
product design, standardized materials, and enhanced clinical validation. This achievement secured a single
medical device registration certificate covering all departments. This means the same product set can be used
compliantly across diverse clinical scenarios, marking a leap from “one certificate per department” to “one
certificate for all departments.”
Industry Significance: Streamlining Processes to Empower Healthcare Efficiency.
Simplified Hospital Procurement and Management: Hospitals no longer need to procure separate products with
distinct certificates for different departments, reducing inventory pressure and procurement costs while significant
ly improving management efficiency.
Enhanced Clinical Flexibility: Emergency rooms, operating rooms, wards, and other settings can rapidly deploy
the same compliant product, shortening preparation time—particularly advantageous during urgent care.
Industry Benchmarking: This initiative advances the standardization and consolidation of medical devices,
establishing a replicable innovative pathway for nationwide registration management of similar products.



